Going Beyond Viral Detection: COVID-19 Diagnostics – TriCon 2021

David Parker, PhD
Senior Vice President, Diagnostics Solutions
The emergence of the COVID-19 pandemic spurred the development of nearly 300 diagnostics aimed at detecting exposure to the SARS-CoV-2 virus. 1 Many of these tests are available for use in multiple settings of care to identify the presence of virus, assess viral load, and determine whether a patient has been infected. As we near the end of the acute epidemiology phase of COVID-19 diagnostics, the next generation of diagnostics will address COVID-19 as an endemic disease.
On February 17, 2021, David Parker, PhD, Senior Vice President of Diagnostic Solutions at Precision for Medicine presented a talk entitled Going Beyond Viral Detection: COVID-19 Diagnostics, at the 28th International Molecular Med Tri-Con Virtual Conference & Expo. As an end-to-end provider of development and commercialization services for advanced diagnostics and therapeutics, Precision for Medicine was honored to have been featured in this international forum.

With the emergence of viral variants and disease etiologies ranging from asymptomatic disease to rapid death, it has become clear that COVID-19 will be an ongoing concern long after the public health emergency comes to an end.
To effectively manage the systemic illness resulting from SARS-CoV-2 infection, appropriate diagnostic tools will be required. In his presentation, Dr. Parker explored the analytes and technologies driving this next phase of COVID-19 research and development.
The Search for Relevant Biomarkers
The Access to COVID-19 Tools (ACT) Accelerator, a global collaborative initiative launched in April 2020, calls for additional molecular tools that can be used to identify relevant biomarkers that address 3 key challenges that go beyond epidemiology.
A wide array of biomarkers for predicting COVID-19 severity and associated complications are currently under investigation (see Table 1). These biomarkers range from the traditional to the novel, including combinations that are being established in research only in the context of COVID-19. All of them are assayed with whole blood, serum, and tissue obtained from patients in various phases of the disease, highlighting the importance of a wide variety of biospecimens from various COVID-19 disease states.
Table 1. Emerging Biomarkers for Predicting COVID-19 Severity2-4

Interleukin-6 (IL-6), an inflammatory biomarker, is prominently featured in many research programs. In June 2020, the Roche Elecsys® IL-6 test received Emergency Use Authorization for identifying patients with confirmed COVID-19 who are at high risk of severe inflammatory response.5 A number of novel cytokines are being examined as well. LIGHT, a cytokine encoded by the TNFS14 gene, has been shown to play a key role in regulating COVID-19 responses in the lung, gut, and skin and may be involved in cytokine storm. Currently, there are also a number of studies-including research in Precision for Medicine's own laboratories-looking at immune cell responses to the disease.
Parker shared an example of a biomarker study measuring trends in C-reactive protein levels as a predictor of COVID-19 respiratory decline. This retrospective cohort study found that trending CRP outperforms respiratory oxygen rate in predicting respiratory deterioration and that CRP levels correlated with IL-6 levels and physiological measures of hypoxemic respiratory failure.6
The Need for Advanced Bioinformatics
Future COVID-19 diagnostics will likely leverage multiple biomarkers and algorithms to associate disease severity. Researchers are applying advanced bioinformatics methods to large data streams encompassing proteomic, immunomic, and genomic analysis to identify not just biomarkers, but biomarker signatures that are prognostic and predictive for COVID-19 disease. Precision for Medicine is supporting the development of one such multi-biomarker assay, Inflammatix's CoVerity™. CoVerity™ is a 5-mRNA classifier test that has demonstrated superiority to clinical biomarkers, including IL-6, in predicting the risk of severe respiratory failure in COVID-19.7
Parker also spoke about MeMed's multianalyte machine learning algorithm for analyzing immune responses to COVID-19, which may have utility in stratifying patients for more aggressive treatment with corticosteroids.8
Next-Generation COVID-19 Diagnostics
The emerging diagnostic clinical need lies in disease management, with a focus on immune system function and activity. Multiple companies have developed cytokine, chemokine, and protein assays as research tools for investigating the COVID-19 disease process and supporting the development of next-generation diagnostics (see Table 2).
Table 2. Molecular Tools for COVID-19 Research
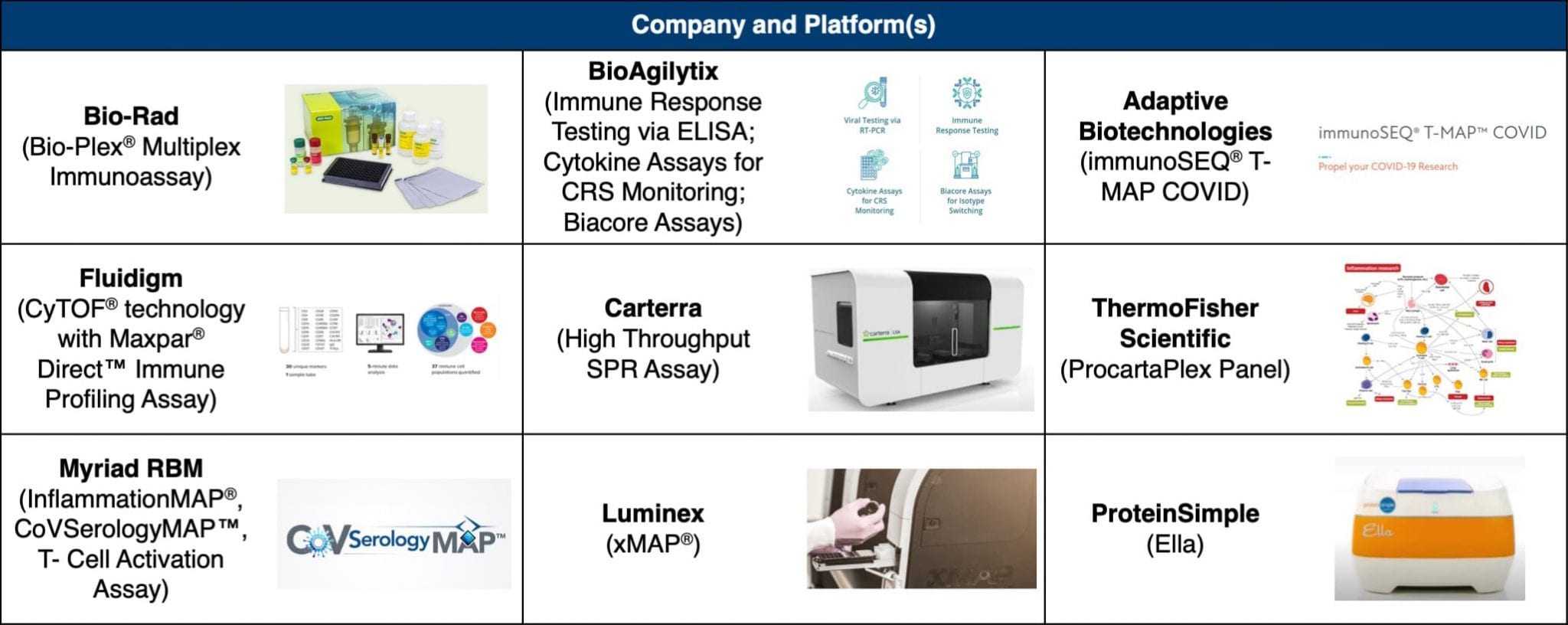
Parker made mention of Fluidigm's immune profiling assay, Luminex's cytokine storm-related biomarker panels, and Adaptive Biotechnologies' quantitative map of T-cell receptors and SARS-CoV-2 antigens.
Precision for Medicine has supported the development and authorization of multiple COVID-19 diagnostics, particularly in the areas of biospecimen sourcing and regulatory strategy. To learn more, watch the full presentation here or visit Precision for Medicine's COVID-19 Resource Center.
1 Biocentury. COVID-19 Diagnostics. Updated January 15, 2021. Accessed February 21, 2021. https://www.biocentury.com/coronavirus/diagnostics.
2 Zhang L, Guo H. Biomarkers of COVID-19 and technologies to combat SARS-CoV-2. Adv Biomark Sci Technol. 2020;2:1-23.
3 Hosseini A, Pandey R, Osman E, et al. Roadmap to the bioanalytical testing of COVID-19: from sample collection to disease surveillance. ACS Sens. 2020;5(11):3328-3345.
4 Tjendra Y, Al Mana AF, Espejo AP, et al. Predicting disease severity and outcome in COVID-19 patients: a review of multiple biomarkers. Arch Pathol Lab Med. 2020;144(12):1465-1474.
6 Mueller AA, Tamura T, Crowley CP, et al. Inflammatory biomarker trends predict respiratory decline in COVID-19 patients. Cell Rep Med. 2020;1(8):100144.
7 Sweeney TE, Liesenfeld O, Wacker J, et al. Validation of inflammopathic, adaptive, and coagulopathic sepsis endotypes in coronavirus disease 2019. Crit Care Med. 2021;49(2):e170-178.
8 Lederfein, et al. Observational cohort study of IP-10's potential as a biomarker to aid in inflammation regulation within a clinical decision support protocol for patients with severe COVID-19. PLoS ONE. 2021;16(1):e0245296.
To learn more about Precision for Medicine’s services, watch the full presentation here, or visit PrecisionForMedicine.com, PrecisionBiospecimens.com, or QuartzBio.com.
