Biospecimens and Biomarkers for Your CDx Strategy – Tri-Con 2021

Rob Fannon, MPH, MBA
General Manager, Biospecimen Solutions, Precision for Medicine
The increasing complexity of immuno-oncology clinical trials heightens the importance of devising a sound strategy for evaluating disease biomarkers as potential trial endpoints or as determinants of eligibility criteria. That places a premium on comprehensive strategic planning to enable biospecimen collection and evaluation as a crucial step in biomarker exploration, particularly with regard to developing potential companion diagnostic (CDx) strategies.
On February 18, 2021, Rob Fannon, General Manager for Biospecimen Solutions at Precision for Medicine, delivered a presentation entitled, Biospecimens and Biomarkers for Your CDx Strategy at the 28th International Molecular Med Tri-Con Virtual Conference & Expo, a leading international forum for the precision medicine community. Precision for Medicine, a global leader in supplying biospecimens, lab services, and CRO services to the life sciences industries, was pleased to have Fannon share his strategic insights with the Tri-Con audience.
Corralling the Movement of Tissue Samples in Immuno-oncology Trials
In a 2016 survey conducted as part of an Applied Clinical Trials webinar, participants rated "highly complex studies" as the most challenging aspect of immuno-oncology clinical trials. A key driver of that complexity is the movement of tissue samples throughout the trial laboratory ecosystem.
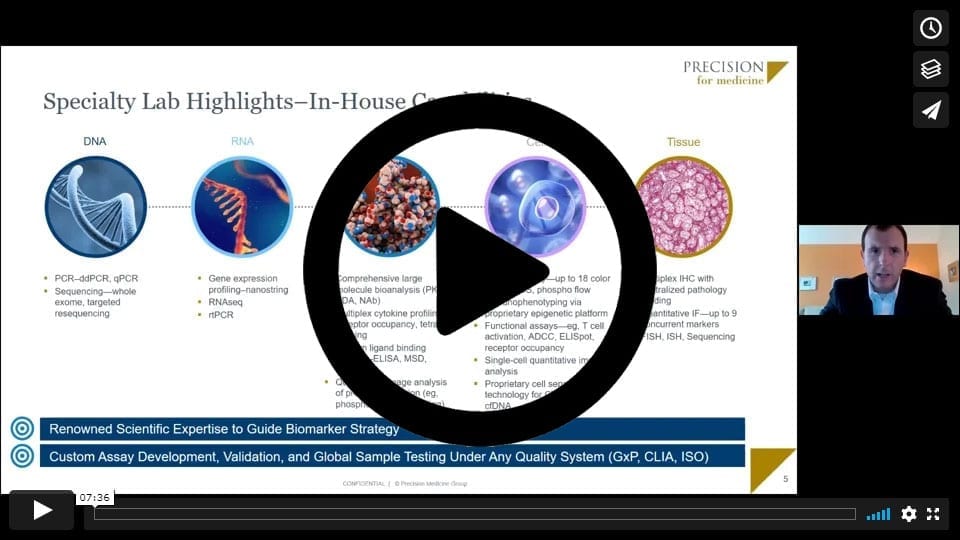
Fannon observed that managing samples across the clinical trial process has never been more complicated than it is today. Immuno-oncology researchers can now choose from among a profusion of sample type assays to evaluate biomarkers as potential exploratory endpoints or to facilitate establishment of inclusion/exclusion criteria. With so many moving parts, precision planning is essential to clinical trial success, as the data generated by these assays is critical to study outcomes, particularly for biomarker-informed clinical trials.
Biospecimen Solutions
Seamless access to the right biospecimens is essential to the biomarker evaluation process. In January 2019, Precision for Medicine established a 70-person Biospecimens and Biorepository group to facilitate custom assay development, validation, and global sample testing for pharmaceutical, biotech, and diagnostics companies. With its in-house bioanalytical capabilities and a full histology suite, Precision for Medicine and its Biospecimens solutions group offers a range of services, including DNA sequencing, RNA gene expression profiling, large-molecule protein bioanalysis, cell immunophenotyping, and multiplex tissue immunohistochemistry (IHC). All of these technologies, across all platforms and analytes, start with biospecimens as a substrate.
Today, the Precision for Medicine Biospecimens group operates at 3 of the company's 7 accredited* laboratories, where samples are ethically sourced from an extensive site network to produce the highest-grade biospecimen products on the market. The bulk of the group's tissue work takes place at a lab founded in 2016 by Dr. Cullen Taylor, a surgical pathologist. The 9,000-square-foot lab, located in Winston-Salem, NC, and staffed by board-certified pathologists, functions as a biorepository with more than 3 million formalin-fixed paraffin-embedded (FFPE) tumor tissues under one roof, with several million histology slides covering a full spectrum of oncologic and medical diseases.
Navigating PD-L1
For any solid tumor clinical development program today, the sponsor must contemplate how to navigate the programmed death-ligand 1 (PD-L1), a protein that has spawned a portfolio of PD-L1 inhibitors that includes nivolumab, pembrolizumab, atezolizumab, and durvalamab. Fannon commented upon the tremendous amount of heterogeneity across the IHC clones that govern PD-L1 testing, noting that IHC "is just as much an art form as it is science." That can make PD-L1 navigation quite daunting, particularly for smaller biotech companies that are wading into the clinical trial ecosystem with a handful of candidates. Those companies must chart how they will run their studies, while also deciding upon which agents and which indications to pursue.
The Strength of a CDx Incubator
Consequently, a growing number of trial sponsors are reaching out to Precision for Medicine at the outset of their programs to secure specimens to assess biomarker prevalence, performance across various testing platforms, and co-expression levels with PD-L1. Such interactions have made the Winston-Salem lab, with its vast histology infrastructure, a veritable "CDx incubator." Even for a CAP/CLIA-ready lab staffed with pathologists, it is rare to have, under one roof, the capability to conduct development work across all major IHC platforms, with an abundant supply of expendable, compliantly collected, research-ready tissues to develop validated assays for clinical trials.
With access to 3 leading IHC platforms, the Winston-Salem lab enables method comparison studies, repeatability and reproducibility, and biomarker evaluation, using the lab's extensive in-house biomarker inventory. These capabilities epitomize Precision for Medicine's approach, starting with the end-use in mind and accommodating scientifically grounded strategies accordingly through the compilation of the right sample sets, fit for purpose for the application.
Figure 1: CDx Incubator for IHC Assays

Figure 2: Complex Biomarker Capabilities From Assay Development Through CDX

*Accredited by the College of American Pathologists (CAP) and certified according to the Clinical Laboratory Improvement Amendments of 1988 (CLIA).
To learn more about Precision for Medicine's services, watch the full presentation here.
